Small Intestinal Foreign Body Guide
Key Points
- Obstruction may be caused by a discrete foreign body (DFB) or a linear foreign body (LFB) and may be partial or complete
- Common clinical signs include lethargy, anorexia, vomiting and abdominal pain
- Diagnosis is via abdominal palpation, radiography or ultrasonography
- Ultrasonography has the highest diagnostic value
- The decision to proceed to surgery will depend upon many factors including the type of foreign body, the problems associated with the material of the foreign body and the degree of obstruction that it causes.
Not all small intestinal foreign bodies require surgical intervention - Prognosis is good with early appropriate surgery
- Without prompt appropriate treatment, there is a risk of decreased bowel perfusion and bowel wall necrosis, septic peritonitis and death
- Increased mortality has been reported for: LFB vs DFB, if multiple enterotomies are performed and with a longer duration of clinical signs
Definition
- Partial or complete obstruction of the small intestine caused by ingestion of foreign material
- Partial or complete obstruction of the small intestine caused by ingestion of foreign material
Prevalence
- Reported prevalence of gastrointestinal foreign bodies in USA hospitals of 26.4 per 10,000 cases (dogs) and 16.1 per 10,000 cases (cats)
- Prevalence from UK first opinion practices indicate that overall gastrointestinal foreign bodies are significantly more common in dogs than in cats
[1][53]
- Prevalence of dogs with gastrointestinal foreign bodies presented to Banfield hospitals (USA) in 2014 was 26.4 per 10,000 cases53
- Prevalence of cats with gastrointestinal foreign bodies presented to Banfield hospitals (USA) in 2014 was 16.1 per 10,000 cases53
- Prevalence data from UK indicate that overall gastrointestinal foreign bodies are significantly more common in dogs than in cats. Out of 208 cases presented with gastrointestinal foreign body to first opinion practice over 48 months, 184 were dogs and 24 were cats1
Causes
- Discrete foreign body entrapment (e.g. plastic toys, stones, balls, glue, trichobezoars, food, cork, nuts, bone, tissue)
- Linear foreign body (e.g. string, nylon stockings, sewing thread, fishing line, cloth, wire, rope)
- The intestine proximal to the obstruction dilates with gas and secretions
- Severe cases may result in ischaemic necrosis of the intestinal wall
[1][2][3][4][5][6][7][72][79][94][97][98]
- Discrete foreign body (DFB) entrapment (e.g. plastic toys, stones, balls, glue, trichobezoars, cork, nuts, bone, tissue)1,2,3,72,79,97,98
- Linear foreign body (LFB) (e.g. string, nylon stockings, sewing thread, fishing line, cloth, wire, rope)1,4,5,79,97,98
- The most common categories of gastrointestinal foreign bodies (not including those with metallic or mineral components) in a U.S. survey were balls, food items, fabric, soft plastic and hard plastic94
- The most common site in the dog is jejunum1,2,6
- Cats appear to have a more uniform distribution of locations1,5
- The intestine proximal to the obstruction dilates with gas and secretions7
- Severe cases may result in ischaemic necrosis of the intestinal wall7
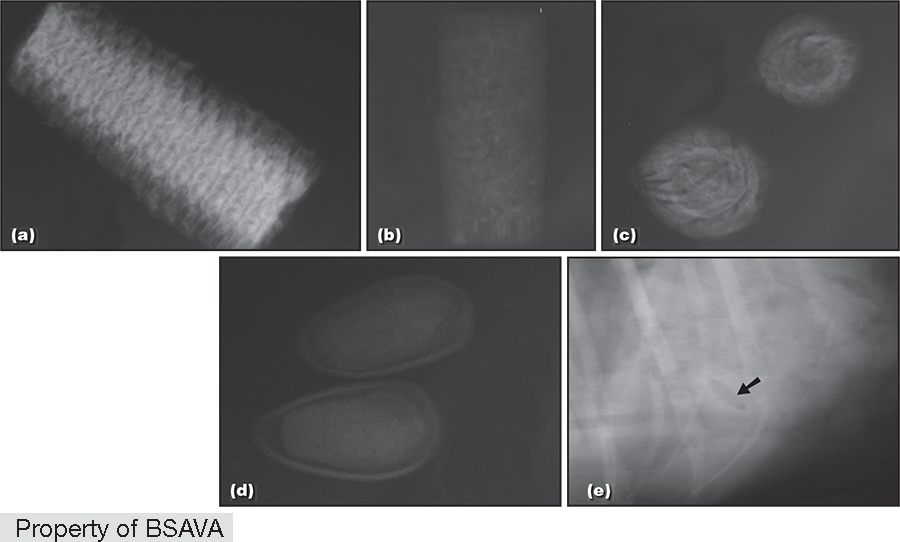
Risk Factors
- Young cats
- Young, medium to large dogs
- Over-represented dog breeds include Terriers and Labrador/Golden Retrievers
- Increased risk of intestinal necrosis and perforation in dogs with increased duration of clinical signs, increased preoperative lactate, presence of a linear foreign body and delayed surgery
[1][6][8][9][10][24][65][87]
- Young cats and young, medium to large-breed dogs are over-represented1,9,10,24,87
- Dog breeds that are over-represented vary depending on the study:
Staffordshire Bull Terrier, English Bull Terrier, Jack Russell Terrier, Border Collie, Springer Spaniel1
Labrador Retriever, Dachshund, and German Shepherd Dog8
Labrador Retriever, Golden Retriever, American Pit Bull Terrier6 - Labrador Retrievers, Mixed Breed dogs, English Bulldogs and Golden Retrievers were over-
represented in a retrospective study of obstructive pyloric and duodenal foreign bodies87 - Increased risk of intestinal necrosis and perforation in dogs with increased duration of clinical signs, increased preoperative lactate, presence of a linear foreign body and delayed surgery > 6hrs65
Clinical Features
- Common clinical signs include lethargy, anorexia, vomiting and abdominal pain
- Diarrhoea may be seen in partial obstructions
- Coughing, hairballs, hiding away and altered urination may be seen in cats
- Protracted vomiting or diarrhoea may lead to dehydration and hypovolaemic shock
- If signs of pyrexia and abdominal distension are present, septic peritonitis should be suspected
- The foreign body may be palpable with the patient conscious or under general anaesthesia but the absence of a palpable foreign body does not exclude the diagnosis
[1][2][3][4][5][6][7][10][15][16][81][96][98]
- The clinical signs seen in animals with small intestinal obstructions vary with the location, duration and severity of the obstruction4,5
- Foreign bodies (or an intestinal abnormality) may be palpable in the conscious patient (detection may improve if anaesthetised or sedated);1,7 however, in one large study in dogs, only 13% of DFB and 15% of LFB were palpable6
- Median duration of clinical signs was longer in cats with DFBs (4 days) versus LFBs (2 days) in a large retrospective study97
Common clinical signs
- Lethargy (61–92%)1,2,3,5,6,15,81,96
- Anorexia 55–85%1,6,65,81,96,98
- Vomiting 87–100%1,6,15,16,65,81,96,98
- Abdominal pain2,5,6,7,81 (more likely with linear foreign bodies)6
- Palpable mass in abdomen81
- If signs of pyrexia and abdominal distension are present, septic peritonitis should be suspected4
- Protracted or profuse vomiting or diarrhoea can result in dehydration and eventually hypovolaemia10
Less common clinical signs
- Diarrhoea 5–23.8%1,81,96,98
May be more common in cases with partial obstruction7 - Haemorrhagic diarrhoea 2%1
- Coughing, hairballs, hiding away and altered urination (cats)81
Investigations
| First-line diagnostics |
|
| Investigations to consider |
|
| Emerging tests |
|
| Haematology |
|
| Biochemistry |
|
| Electrolytes and acid–base balance |
|
| Abdominal radiography |
Dog
Cats
|
| Abdominal ultrasoography |
|
| Computed tomography (CT) |
|
| Peritoneal fluid analysis |
|
| Intestinal fatty acid binding protein (I‐FABP) |
|
| Haematology |
| ||||||||||||||
| Biochemistry |
| ||||||||||||||
| Electrolytes and acid–base balance |
| ||||||||||||||
| Abdominal radiography |
Dogs
A 2014 study12 used measurements of L5 body height at its narrowest point (L5), maximum small intestinal diameter (SImax), minimum small intestinal diameter (SImin) and average small intestinal diameter (SIave)
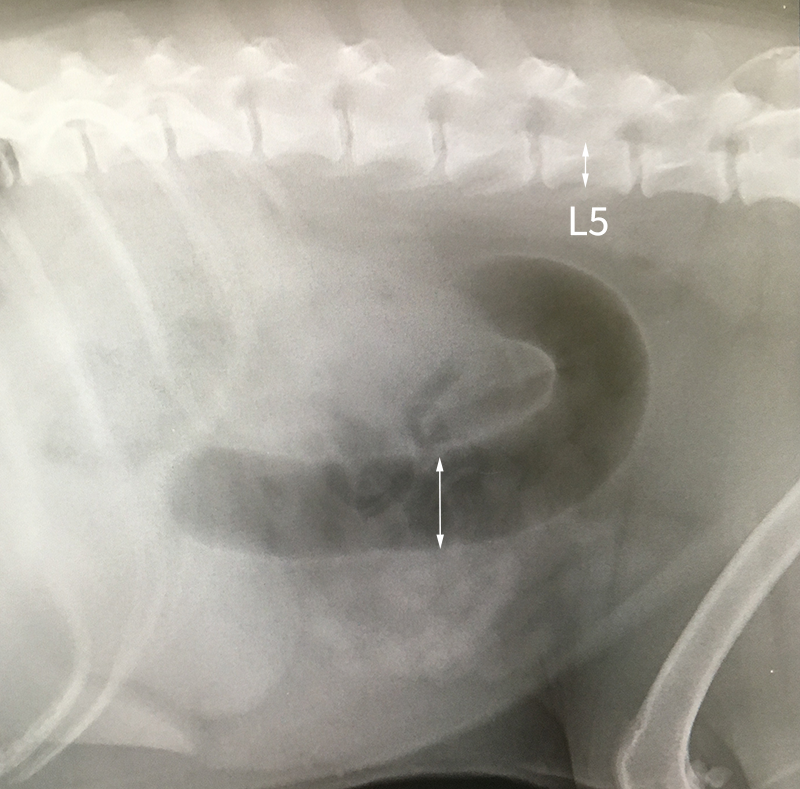 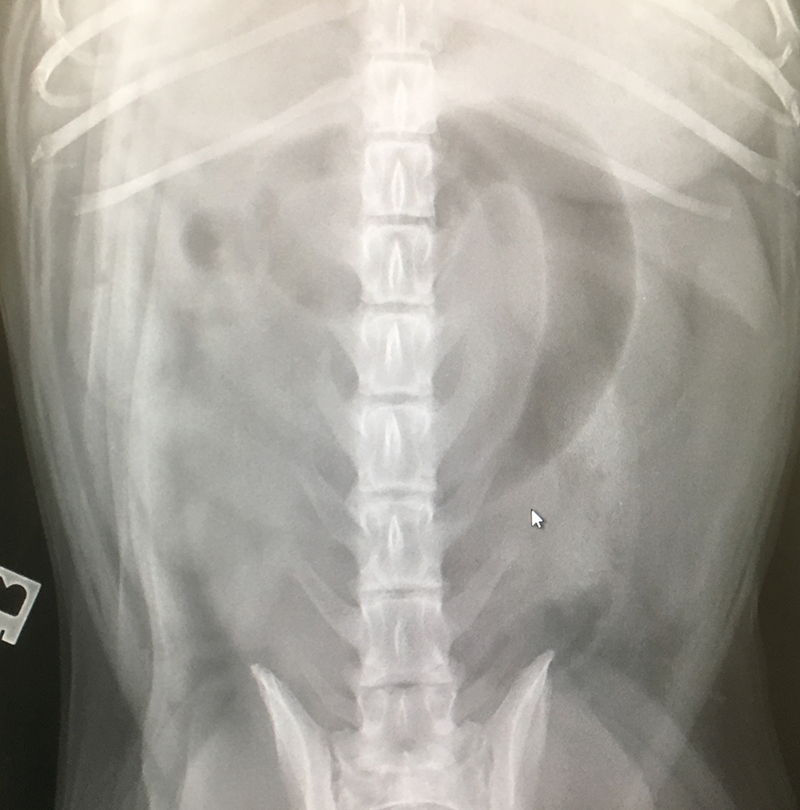 Cats
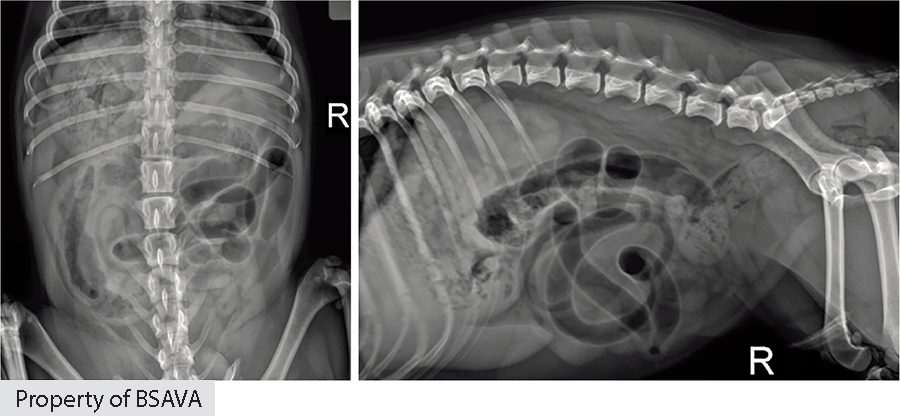 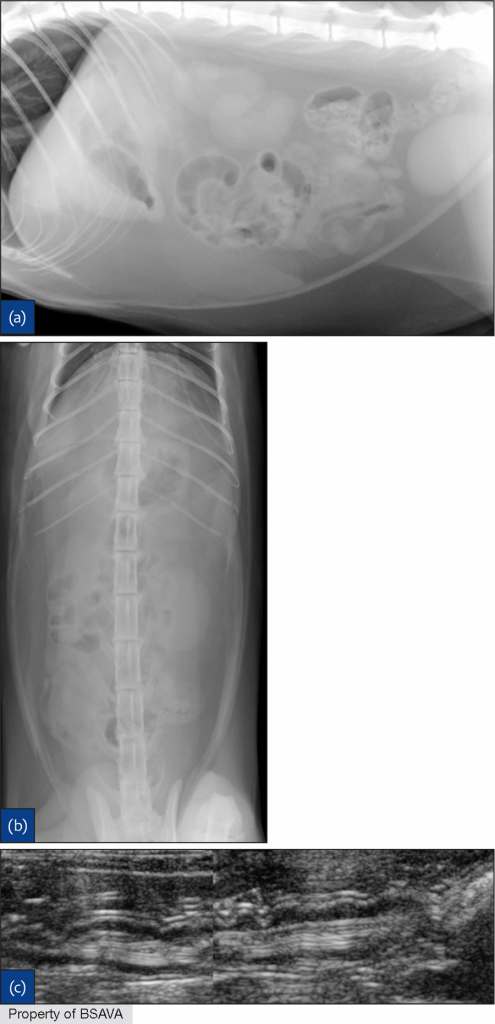 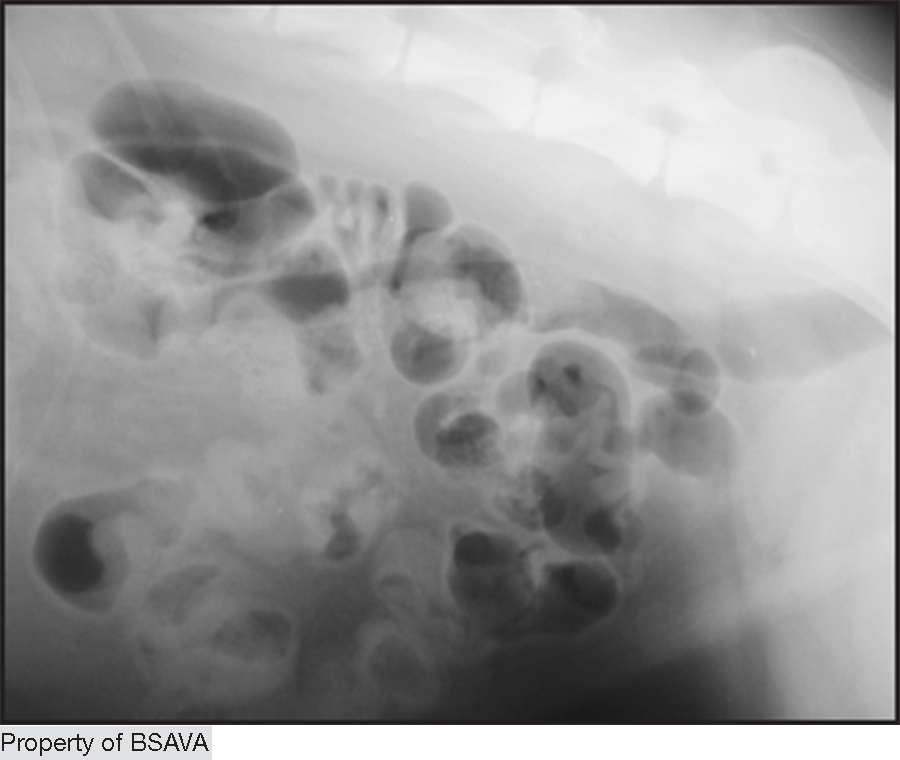 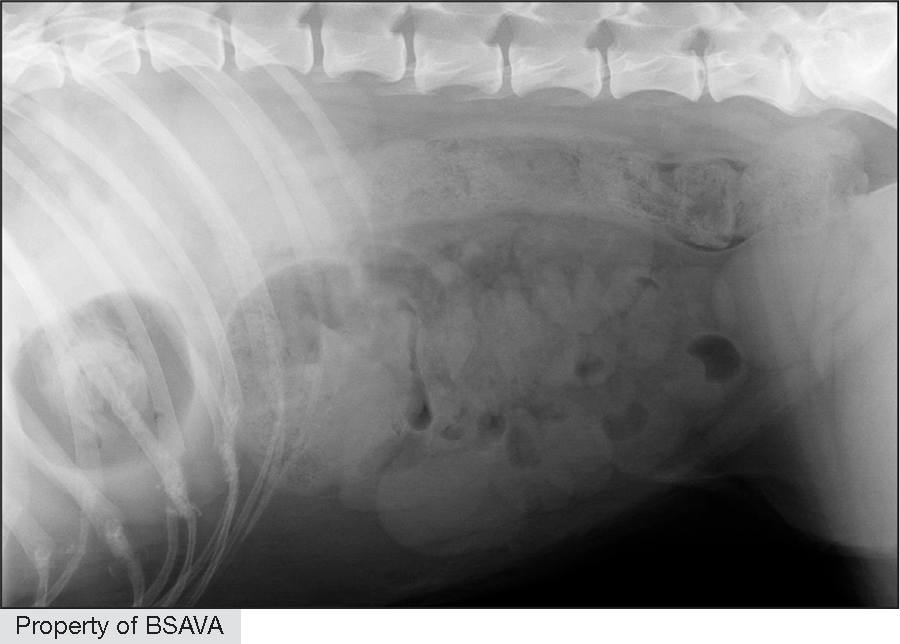 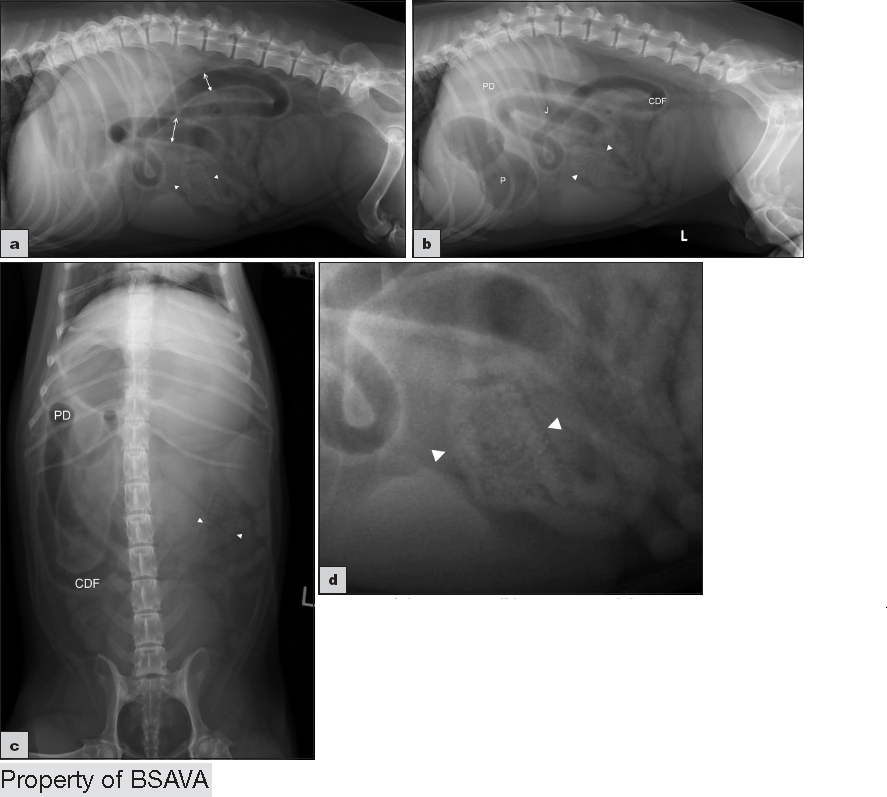  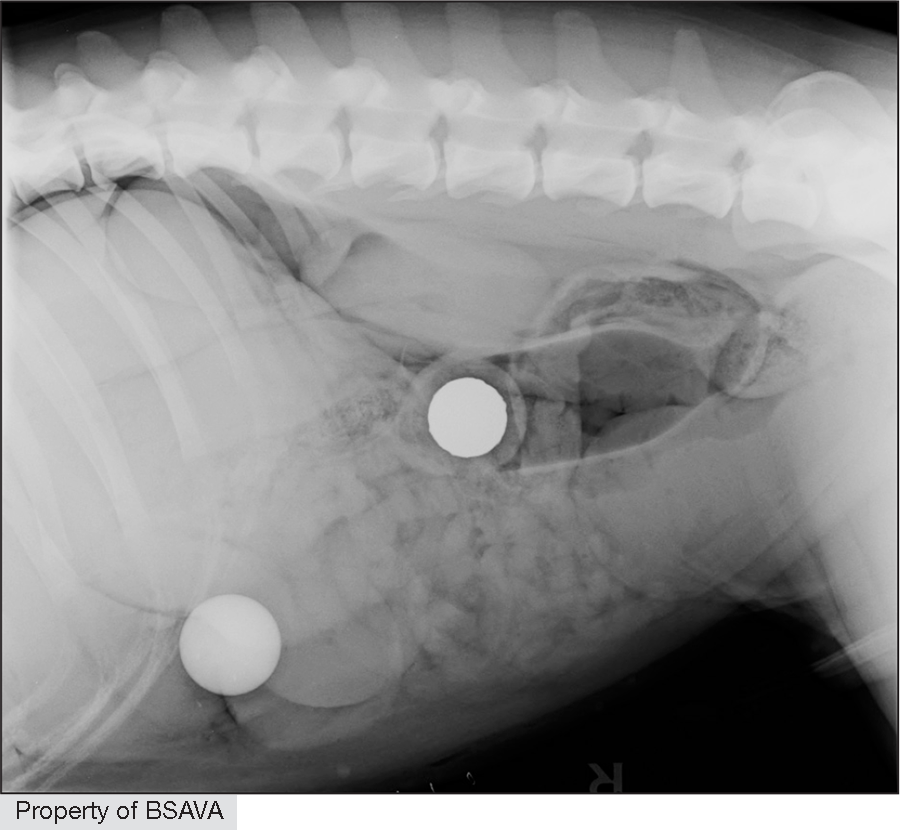   | ||||||||||||||
| Abdominal ultrasonography |
   | ||||||||||||||
| Computed tomography (CT) |
| ||||||||||||||
| Peritoneal fluid analysis | Peritoneal fluid cytology
Peritoneal fluid nucleated cell count
Peritoneal fluid biochemistry
| ||||||||||||||
| Intestinal fatty acid binding protein (I‐FABP) |
|
Diagnosis
- High degree of clinical suspicion based on signalment and presenting signs
- Diagnosis with abdominal palpation, abdominal radiography or ultrasonography
- High degree of clinical suspicion based on signalment and presenting signs
- Diagnosis can be made by abdominal palpation or with abdominal radiography or ultrasonography
Differential Diagnosis
- Gastric foreign body
- Neoplasia
- Intussusception
- Drug side effects
- Addison’s disease
- Toxin ingestion
- Viral infection
- Gastroenteritis
- Pancreatitis
- CAUTION Misdiagnosing functional ileus as mechanical obstruction (or vice versa) can lead to unnecessary surgery or delayed intervention, so combining history, clinical exam, and imaging (especially serial radiographs or ultrasound) is key
[55]
Differential diagnoses55
- Gastric foreign body
- Neoplasia
- Intussusception
- Drug side effects
- Addison’s disease
- Toxin ingestion
- Viral infection
- Gastroenteritis
- Pancreatitis
- Mesenteric volvulus
- CAUTION Misdiagnosing functional ileus as mechanical obstruction (or vice versa) can lead to unnecessary surgery or delayed intervention, so combining history, clinical exam, and imaging (especially serial radiographs or ultrasound) is key
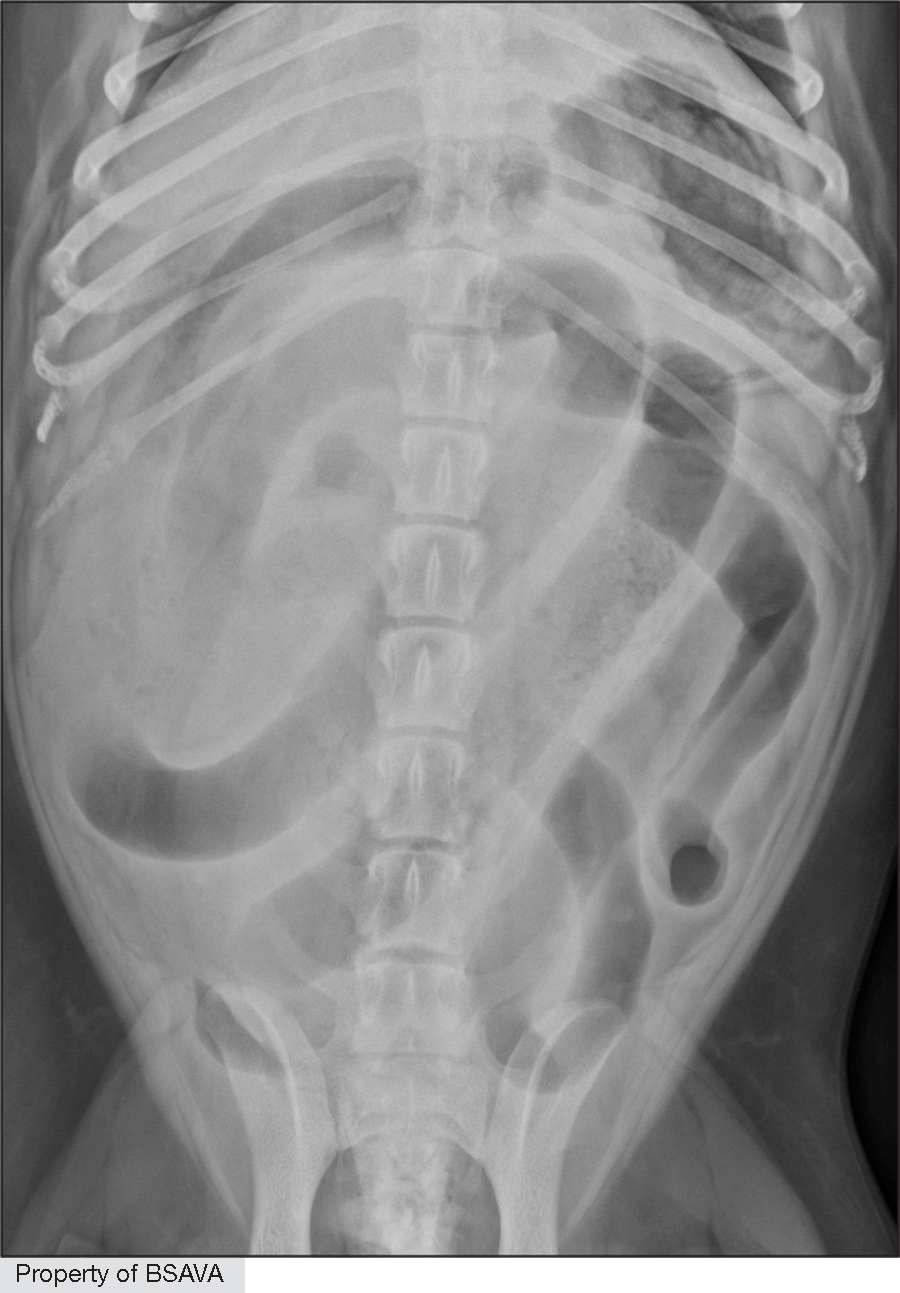
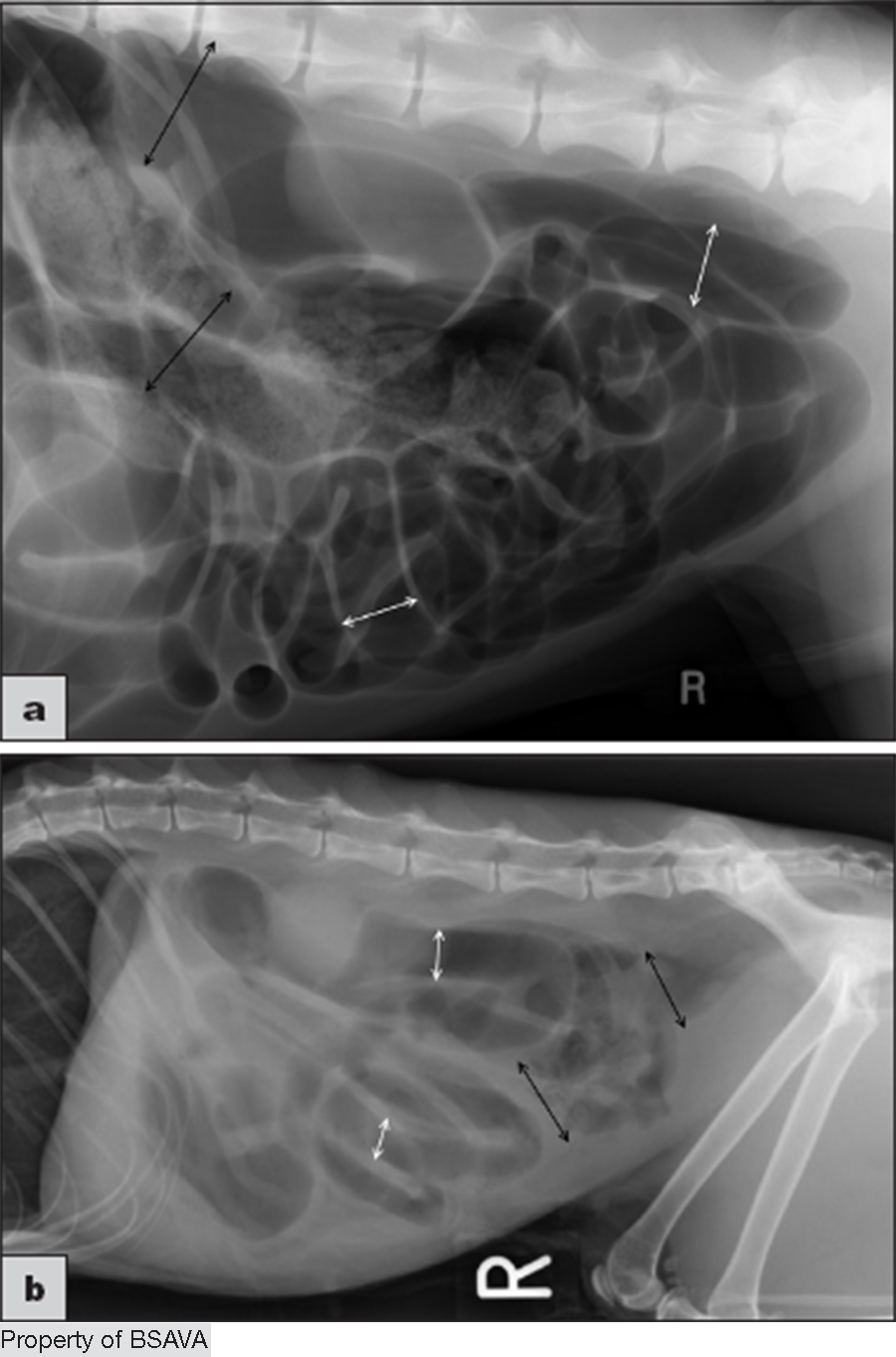
Treatment
| Treatment plan |
|
| Fluid therapy |
|
| Analgesia |
|
| Antibiotics |
|
| Use of antiemetics? |
|
| General principles for enterotomy or enterectomy |
|
| Alternative to enterotomy |
|
| Assessment of viability |
|
| Discrete foreign body |
|
| Linear foreign body |
|
| Enterectomy and anastomoses | |
| Postoperative care |
|
| Emerging therapies |
|
| Treatment plan |
|
| Fluid therapy |
|
| Analgesia |
|
| Antibiotics |
|
| Use of antiemetics? |
|
| General principles for enterotomy or enterectomy |
Suture pattern
Suture material
Stapling
Leak testing
Probe Testing
Omental wrapping
Before closure
Risks for dehiscence
 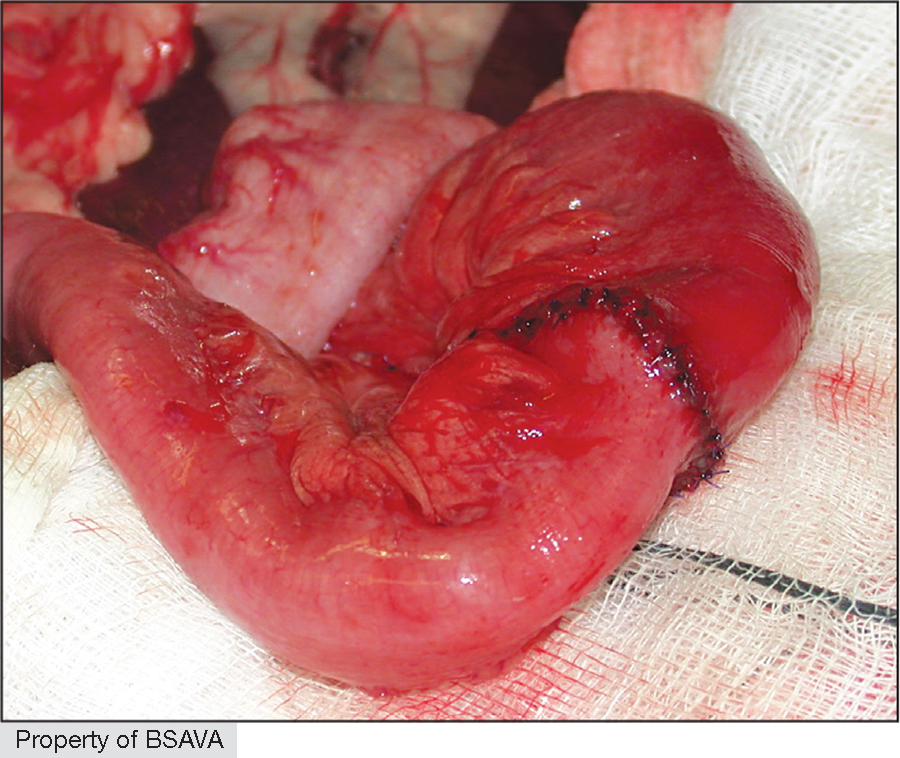 |
| Alternative to enterotomy |
|
| Assessment of viability |
|
| Discrete foreign body | Discrete foreign body (DFB)
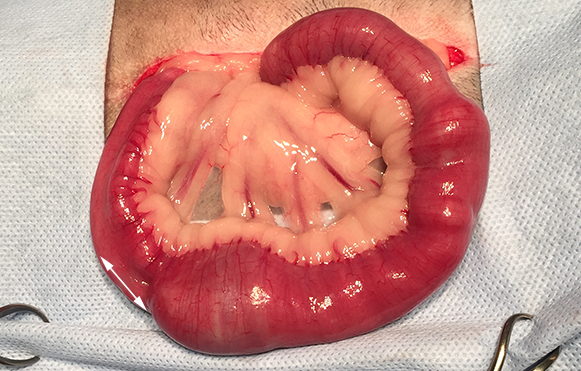 |
| Linear foreign body | Linear foreign body (LFB)
Red rubber catheter technique (RRCT)
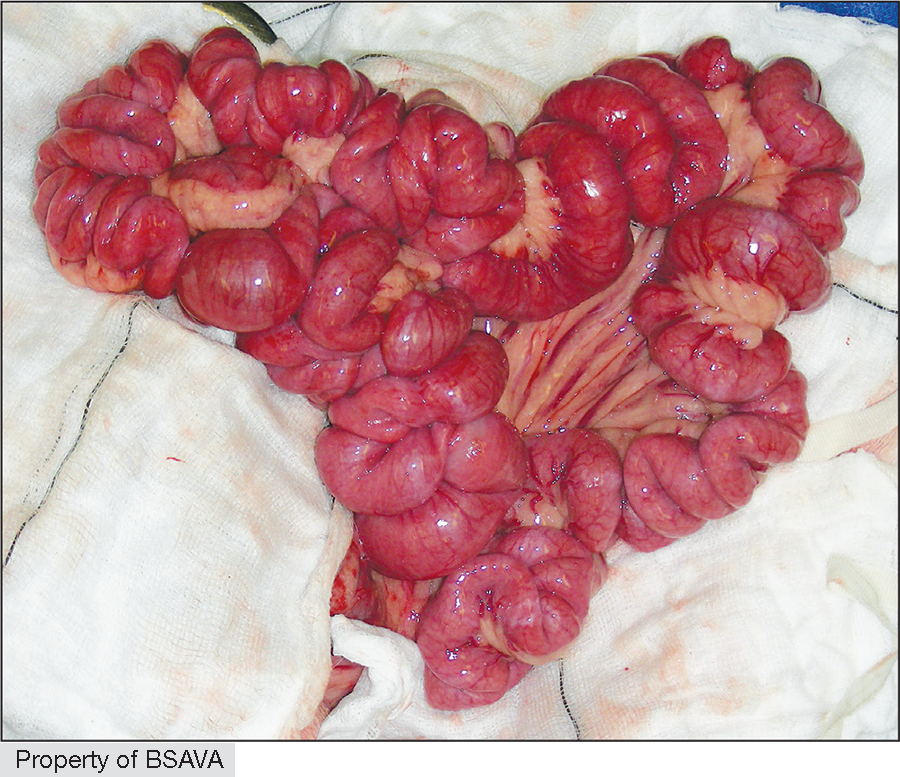 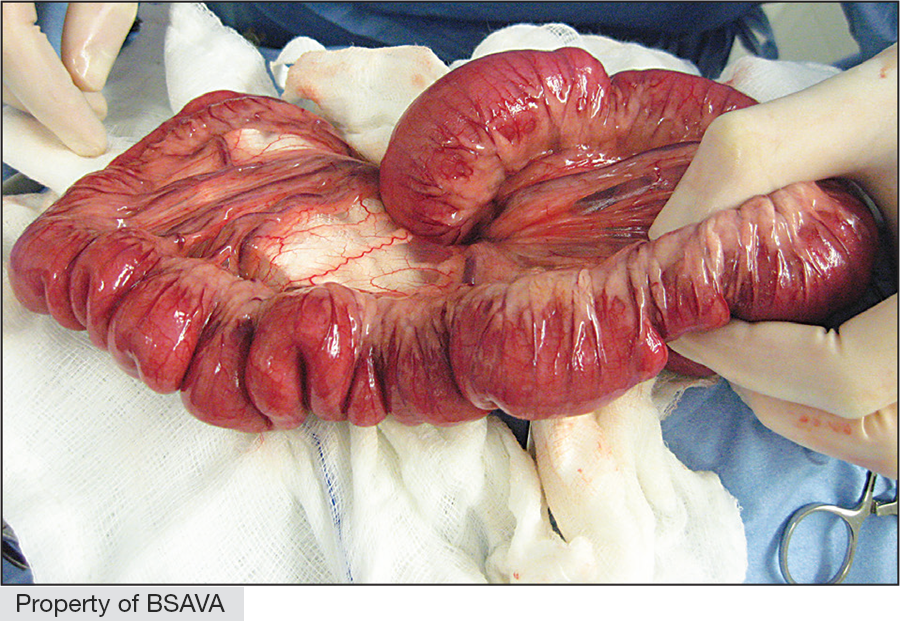 |
| Enterectomy and anastomoses | |
| Postoperative care |
|
| Emerging therapies | Canine-specific albumin (CSA) / Lyophilised canine albumin (LCA)
Dose
|
Complications
- Complications include intestinal injury requiring resection/anastomosis, surgical site infection, peri-operative hypotension, septic peritonitis, incisional dehiscence, intestinal adhesions, postoperative ileus/pancreatitis, short bowel syndrome, aspiration pneumonia, acute respiratory distress syndrome, post-operative hypo/hyperthermia, vomiting/regurgitation, surgical site infection or dehiscence, zinc toxicosis, myocardial injury and death
[1][6][8][10][20][36][49][52][81][84][88][97][98]
- Intestinal injury requiring resection/anastomosis e.g. serosal tear84,97,98
- Bowel wall necrosis, septic peritonitis and death1
- Peri-operative hypotension97
- Intestinal adhesions97
- Incisional dehiscence8,20,36,49,97
- Postoperative ileus49,98
- Postoperative pancreatitis10,98
- Aspiration (pneumonia)10,84,97,98
- Acute respiratory distress syndrome6
- Post-operative hypothermia97
- Post-operative hyperthermia97
- Vomiting or regurgitation97
- Short bowel syndrome (diarrhoea, weight loss) caused by extensive resection. This is rare even if > 50% of the intestine is resected and is usually only transient52
- Surgical site infection98 (may be more common following LFB surgery)81
- Surgical site dehiscence98
- Zinc toxicosis – caused by metal foreign bodies containing zinc. This may manifest as haemolytic anaemia, acute liver injury, coagulopathy, thrombocytopenia, AKI and acute pancreatitis88
- Myocardial injury98
- Death84
Incidence of complications
- In a large retrospective feline study, 34/126 cats experienced minor post-operative complications (hyperthermia, hypothermia, surgical site infections, regurgitation, aspiration pneumonia and vomiting)97
- In cats with DFB, 4/21 experienced major post-op complications (dehiscence, death due to SIRS, euthanasia)97
- There was no difference in the frequency of complications between cats with a DFB compared with those with an LFB97
Prognosis
- Prognosis is good with early, appropriate surgery
- Without prompt treatment, there is a risk of decreased bowel perfusion and bowel wall necrosis, septic peritonitis and death
- Reported survival for pets with DFB (dogs 94–96%, cats 100%)
- Reported survival for LFB (dogs 80-96%, cats 63-100%)
- Reported dehiscence rates are 2-3.8% (enterotomies) and 14-18.2% (enterectomies)
- Increased risk of dehiscence if hypoproteinaemia, preoperative peritonitis, multiple gastrointestinal incisions, the presence of a LFB, ASA score >3 and an older age
- Implementation of a structured perioperative Veterinary Enhanced Recovery After Surgery (Vet-ERAS) protocol in dogs undergoing emergency laparotomy showed promise for improving short-term mortality and complications in dogs undergoing exploratory laparotomy.
[1][2][5][6][7][8][21][27][37][67][81][97][100][101]
- Prognosis is good with early appropriate surgery5,7,81
- Without prompt treatment, there is a risk of decreased bowel perfusion and bowel wall necrosis, septic peritonitis and death1
- Reported survival for pets with DFB (dogs 94–96%,1,6 cats 100%1,81)
- Reported survival for LFB (dogs 80-96%1,6, cats 63-100%1,81)
- Increased mortality has been reported when multiple enterotomies are required/performed1, with a longer duration of clinical signs1,2 and for LFB vs DFB in a charity hospital;1 however, in a referral population, 96% of dogs survived to hospital discharge, with no difference in dogs with linear and nonlinear foreign bodies6
- Reported dehiscence rates are 2-3.8%8,67(enterotomy) and 14-18.2%37,67(enterectomy) with an overall dehiscence rate for both surgeries of 6.6%67
- Increased risk of dehiscence associated with hypoproteinaemia (serum albumin < or equal to 25 g/l),27,100 preoperative peritonitis,27 multiple gastrointestinal incisions21 and the presence of a LFB21 ASA score > 3 and an older age (for each year increase in age, the odds of dehiscence increased by 1.24)67
- A large retrospective study of cats with linear and discrete foreign bodies found that 166/169 survived surgery. Survival to discharge was not affected by foreign body type (DFB versus LFB). All of the cats that had follow-up data available (n=126) were alive at 2 weeks post-op.97
- In an initial prospective study of 59 dogs, a structured peri-operative Veterinary Enhanced Recovery After Surgery (Vet-ERAS) protocol showed promise for improving short-term mortality and complications in dogs undergoing exploratory laparotomy. Further studies are needed to validate this result as control and study groups were not directly comparable. The Vet-ERAS protocol includes simple pre-operative stabilisation, intra-operative and postoperative steps. These aim to optimise communication, fluid balance, infection control, pain relief, early nutrition and mobilisation to achieve better clinical outcomes.101
Discrete foreign bodies (DFB)
- Reported survival for pets with DFB (dogs 94–96%,1,6 cats 100%1,81)
Linear foreign bodies (LFB)
- Reported survival for LFB (dogs 80-96%1,6, cats 63-100%1,81)
- Increased mortality has been reported when multiple enterotomies are required/performed1, with a longer duration of clinical signs1,2 and for LFB vs DFB in a charity hospital;1 however, in a referral population, 96% of dogs survived to hospital discharge, with no difference in dogs with linear and nonlinear foreign bodies6
- A study comparing 56 cats with LFB vs DFB found that LFB cats had:81
Higher body condition scores81
Higher albumin81
Longer surgery time81
Higher ASA scores81
Higher total costs81
Higher rates of surgical site infection81
They also required more intensive post-operative care. However, survival rates did not significantly differ between LFB and DFB groups81
References
1. Hayes, G. (2009)
Gastrointestinal foreign bodies in dogs and cats: a retrospective study of 208 cases
Journal of Small Animal Practice; 50 (11 ) 576–583
Abstract
2. Capak, D., Brkić, A., Harapin, I., Maticic, D. and Radišić, B. (2001)
Treatment of the foreign body induced occlusive ileus in dogs
Veterinarsiki Archiv; 71 (6) 345-359
Abstract
3. Barrs, V.R., Beatty, J.A., Tisdall, P.L. et al. (1999)
Intestinal obstruction by trichobezoars in five cats
The Journal of Feline Medicine and Surgery; 1 (4) 199-207
Abstract
4. Aronson, L.R., Brockman, D.J. and Brown, D.C. (2000)
Gastrointestinal emergencies
Veterinary Clinics of North America: Small Animal Practice; 30 (3) 555-579
Abstract
5. Bebchuk, T.N. (2002)
Feline gastrointestinal foreign bodies
Veterinary Clinics of North America: Small Animal Practice; 32 (4) 861-880
Abstract
6. Hobday, M.M., Pachtinger, G.E., Drobatz, K.J and Syring, R.S. (2014)
Linear versus non-linear gastrointestinal foreign bodies in 499 dogs: clinical presentation, management and short-term outcome
Journal of Small Animal Practice; 55 (11) 560-565
Abstract
7. Papazoglou, L. and Rallis, T.S. (2003)
Intestinal Foreign Bodies in Dogs and Cats
Compendium; 25 (11) 830-843
Full text avalable
8. Strelchik, A., Coleman, M.C., Scharf, V.F., et al. (2019)
Intestinal incisional dehiscence rate following enterotomy for foreign body removal in 247 dogs
Journal of the American Veterinary Medical Association; 255 (6) 695-699
Abstract
9. Aronson, L. R. (2016).
Small Animal Surgical Emergencies
Chapter 4. Gastrointestinal Foreign Bodies
Wiley Blackwell. p 33-41
10. Boag, A.K., Coe, R. J., Martinez, T. A. and Hughes, D. (2005)
Acid-Base and Electrolyte Abnormalities in Dogs with Gastrointestinal Foreign Bodies
Journal of Veterinary Internal Medicine; 19 (6) 816–821
Full text available
11. Graham, J.P., Lord, P.F. and Harrison, J.M. (1998)
Quantitative estimation of intestinal dilation as a predictor of obstruction in the dog
Journal of Small Animal Practice; 39 (11) 521-524
Abstract
12. Finck, C., D’Anjou, M.A., Alexander, K., Specchi, S. and Beaucham,p G. (2014)
Radiographic diagnosis of mechanical obstruction in dogs based on relative small intestinal external diameters
Veterinary Radiology and Ultrasound; 55 (5) 472-479
Abstract
13. Adams, W.M., Sisterman, L.A., Klauer, J.M., Kirby, B.M. and Lin, T.L. (2010)
Association of intestinal disorders in cats with findings of abdominal radiography
Journal of the American Veterinary Medical Association; 236 (8) 880-886
Abstract
14. Morgan, J.P. (1981)
The upper gastrointestinal examination in the cat: Normal radiographic appearance using positive contrast medium
Veterinary Radiology and Ultrasound; 22 (4) 159-169
Abstract
15. Tyrrell, D. and Beck, C. (2006)
Survey of the use of radiography vs ultrasonography in the investigation of gastrointestinal foreign bodies in small animals
Veterinary Radiology and Ultrasound; 47 (4) 404–408
Abstract
16. Sharma, A., Thompson, M. S., Scrivani, P. V., et al. (2011)
Comparison of radiography and ultrasonography for diagnosing small-intestinal mechanical obstruction in vomiting dogs
Veterinary Radiology and Ultrasound; 52 (3) 248-255
Abstract
17. Matthews, K., Kronen, P.W., Lascelles, D. et al. (2014)
Guidelines for Recognition, Assessment and Treatment of Pain
Journal of Small Animal Practice; 55 (6) E10-E68
Full text available
18. Ramsey I. (2017)
Small Animal Formulary. Part A: Canine and Feline
BSAVA 9th edition
19. Weisman, D.L., Smeak, D.D., Birchard, S.J. and Zweigart, S.L. (1999)
Comparison of a continuous suture pattern with a simple interrupted pattern for enteric closure in dogs and cats: 83 cases (1991-1997)
Journal of the American Veterinary Medical Association; 214 (10) 1507-1510
Abstract
20. Sumner, S.M., Regier, P.J., Case, J.B. and Ellison, G.W. (2019)
Evaluation of suture reinforcement for stapled intestinal anastomoses: 77 dogs (2008‐2018)
Veterinary Surgery; 48 (7) 1188-1193
Abstract
21. Schwartz, Z. and Coolman, B.R. (2018)
Disposable skin staplers for closure of linear gastrointestinal incisions in dogs
Veterinary Surgery; 47 (2) 285-292
Abstract
22. Kieves, N.R., Krebs, A.I. and Zellner, E.M. (2018)
A Comparison of Ex Vivo Leak Pressures for Four Enterotomy Closures in a Canine Model
Journal of the American Veterinary Medical Association; 54 (2) 71-76
Abstract
23. Ellison, G.W., Case, J.B. and Regier, P.J. (2019)
Intestinal surgery in small animals: historical foundations, current thinking, and future horizons
Veterinary Surgery; 48 (7) 1171-1180
Abstract
24. Evans, K.L., Smeak, D.D. and Biller, D.S. (1994)
Gastrointestinal linear foreign bodies in 32 dogs: a retrospective evaluation and feline comparison
Journal of the American Veterinary Medical Association; 30 (5) 445-450
Abstract
25. Anderson, S., Lippincott, C.L. and Gill, P.G. (1992)
Single enterotomy removal of gastrointestinal linear foreign bodies
Journal of the American Veterinary Medical Association; 28 (6) 487–490
26. Gosling, M.J. and Martínez-Taboada, F. (2018)
Adverse reactions to two intravenous antibiotics (Augmentin and Zinacef) used for surgical prophylaxis in dogs
Veterinary Record; 182 (3) 80
Abstract
27. Grimes, J. A., Schmiedt, C. W., Cornell, K. K. et al. (2011)
Identification of risk factors for septic peritonitis and failure to survive following gastrointestinal surgery in dogs
Journal of the American Veterinary Medical Association; 238 (4) 486-494
Abstract
28. Ohno, T., Mochiki, E., Ando, H. et al. (2009)
Glutamine decreases the duration of postoperative ileus after abdominal surgery: an experimental study of conscious dogs
Digestive Diseases and Sciences; 54 (6) 1208–1213
Abstract
29. Bonczynski, J.J., Ludwig, L.L., Barton, L.J., Loar, A. and Peterson, M.E. (2003)
Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats
Veterinary Surgery; 32 (2) 161-166
Abstract
30. Levin, G.M., Bonczynski, J.J., Ludwig, L.L., Barton, L.J. and Loar, A.S. (2004)
Lactate as a diagnostic test for septic peritoneal effusions in dogs and cats
Journal of the American Veterinary Medical Association; 40 (5) 364-371
Abstract
31. Ciasca, T.C., David, F.H. and Lamb, C.R. (2013)
Does measurement of small intestinal diameter increase diagnostic accuracy of radiography in dogs with suspected intestinal obstruction?
Veterinary Radiology and Ultrasound; 54 (3) 207-211
Abstract
32. Sun, D., Cen, Y., Li, S. et al. (2016)
Accuracy of the serum intestinal fatty-acid-binding protein for diagnosis of acute intestinal ischemia: a meta-analysis
Scientific Reports; 6 34371
Full text available
33. Parratt, C., Harrand, R., Moody, E. et al. (2019)
Plasma Concentrations of Intestinal Fatty Acid Binding Protein, Vasoactive Intestinal Polypeptide, C‐Reactive Protein, and Interleukin‐6 in Vomiting Dogs With and Without Foreign Body Induced Gastrointestinal Obstruction
Journal of Veterinary Emergency and Critical Care; 29 (S1) S2–S50
IVECC, EVECC, ACVECC VetCOT Conference procs.
Full text available
34. Felts, J.F., Fox, PR. and Burk, R.L. (1984)
Thread and sewing needles as gastrointestinal foreign bodies in the cat: a review of 64 cases
Journal of the American Veterinary Medical Association; 184 (1) 56-59
Abstract
35. Coolman, B.R., Ehrhart, N., Pijanowski, G., Ehrhart, E.J. and Coolman, S.L. (2000)
Comparison of skin staples with sutures for anastomosis of the small intestine in dogs
Veterinary Surgery; 29 (4) 293-302
Abstract
36. DePompeo, C.M., Bond, L., George, Y.E. e al. (2018)
Intra-abdominal complications following intestinal anastomoses by suture and staple techniques in dogs
Journal of the American Veterinary Medical Association; 253 (4) 437-443
Full text available
37. Duell, J.R., Thieman Mankin, K.M., Rochat, M.C. et al. (2016)
Frequency of Dehiscence in Hand-Sutured and Stapled Intestinal Anastomoses in Dogs
Veterinary Surgery; 45 (1) 100-103
Abstract
38. Davis, D.J., Demianiuk, R.M., Musser, J., Podsiedlik, M. and Hauptman, J. (2018)
Influence of preoperative septic peritonitis and anastomotic technique on the dehiscence of enterectomy sites in dogs: A retrospective review of 210 anastomoses
Veterinary Surgery; 47 (1) 125-129
Abstract
39. Williams, J. (2014)
Feline Gastrointestinal Surgery Principles and Essential Techniques
Journal of Feline Medicine and Surgery; 16 (3) 231–239
Full text available
40. Saile, K., Boothe, H.W. and Boothe, D.M. (2010)
Saline volume necessary to achieve predetermined intraluminal pressures during leak testing of small intestinal biopsy sites in the dog
Veterinary Surgery; 39 (7) 900-903
Abstract
41. Berríos-Torres, S.I., Umscheid, C.A., Bratzler, D.W. et al (2017)
Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection
Journal of American Medical Association;; 152 (8) 784-791
Full text available
42. Albarellos, G.A. , Montoya, L., Lorenzini, P.M. et al. (2016)
Pharmacokinetics of cefuroxime after intravenous, intramuscular, and subcutaneous administration to dogs
Journal of Veterinary Pharmacology and Therapeutics; 39 (1) 40-44
Abstract
43. Williams, J. (2018)
The Why and How of Antimicrobial Prophylaxis
Companion; 2018 (11) 4-7
Full text available
44. Bratzler, D. W., Houck, P. M.; Surgical Infection Prevention Guideline Writers Workgroup (2005)
Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection
Prevention Project
American Journal of Surgery;189 (4) 395–404
Abstract
45. Shu, X. L., Kang, K., Gu, L. J. and Zhang, Y. S. (2016)
Effect of early enteral nutrition on patients with digestive tract surgery: A meta-analysis of randomized controlled trials
Experimental and Therapeutic Medicine; 12 (4) 2136–2144
Full text available
46. Whitehead, K., Cortes, Y. and Eirmann, L. (2016)
Gastrointestinal dysmotility disorders in critically ill dogs and cats
Journal of Veterinary Emergency and Critical Care; 26 (2) 234-53
Full text available
47. PROTECT ME Antibacterial Guidelines (2018)
BSAVA and SAMSoc
48. Moores, A. (2015)
The Small Intestine
BSAVA Manual of Canine and Feline Abdominal Surgery
2nd edition. Ed. by Williams, J.M. and Niles, J.D.
49. Ellison, G. (2011).
Complications of Gastrointestinal Surgery in Companion Animals
The Veterinary Clinics of North America. Small Animal Practice; 41 (5) 915-934
Abstract
50. Moss, G., Greenstein, A., Levy, S. and Bierenbaum, A. (1980)
Maintenance of GI function after bowel surgery and immediate enteral full nutrition. I. Doubling of canine colorectal anastomotic bursting pressure and intestinal wound mature collagen content
Journal of Parenteral Enteral Nutrition; 4 (6) 535-538
Abstract
51. De-Souza, D.A. and Greene, L.J. (2005)
Intestinal permeability and systemic infections in critically ill patients: effect of glutamine
Critical Care Medicine; 33 (5) 1125-1135
Abstract
52. Gorman, S.C., Freeman, L.M., Mitchell, S.L. et al (2006)
Extensive small bowel resection in dogs and cats: 20 cases (1998-2004)
Journal of the American Veterinary Medical Association; 228 (3) 403–407
Abstract
53. Banfield Hospital (2015)
Prevalence of Gastrointestinal Foreign Bodies
Todays Veterinary Practice; pp. 20
Full text available
54. Mathews, K.A. (2000)
Non Steroidal Anti-inflammatory Analgesics. Indications and Contraindications for Pain Management in Dogs and Cats
Veterinary Clinics of North America: Small Animal Practice; 30 (4) 783-804
Abstract
55. Tams, T.R. (2003)
The Vomiting Dog – Diagnosis
Proceedings. Atlantic Coast Veterinary Conference
Full text available
56. Davis, H., Jensen, D. H., Johnson, T., Knowles, A. et al. (2013)
2013 AAHA/AAFP Fluid Therapy Guidelines for Dogs and Cats
Journal of the American Veterinary Medical Association; 49 (3) 149-158
Full text available
57. Ozben, V., Aytac, E., Liu, X. and Ozuner, G. (2016)
Does omental pedicle flap reduce anastomotic leak and septic complications after rectal cancer surgery?
International Journal of Surgery; 27 53‐57
Full text available
58. Merad, F., Hay, J.M., Fingerhut, A., et al. (1998)
Omentoplasty in the prevention of anastomotic leakage after colonic or rectal resection: a prospective randomized study in 712 patients. French Associations for Surgical Research.
Annals of Surgery; 227 (2) 179‐186
Full text available
59. Ralphs, S.C., Jessen, C.R. and Lipowitz, A.J. (2003)
Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991-2000)
Journal of American Veterinary Medical Association; 223 (1) 73‐77
Abstract
60. White, R.N. (2008)
Modified functional end‐to‐end stapled intestinal anastomosis: technique and clinical results in 15 dogs
Journal of Small Animal Practice; 49 (6) 274-281
Abstract
61. Jardel, N., Hidalgo, A., Leperlier, D., et al (2011)
One stage functional end-to-end stapled intestinal anastomosis and resection performed by nonexpert surgeons for the treatment of small intestinal obstruction in 30 dogs
Veterinary Surgery; 40 (2) 216‐222
Abstract
62. Rosenbaum, J.M., Coolman, B.R., Davidson, B.L., et al. (2016)
The use of disposable skin staples for intestinal resection and anastomosis in 63 dogs: 2000 to 2014
Journal of Small Animal Practice; 57 (11) 631‐636
Abstract
63. Benlloch-Gonzalez, M., Gomes, E., Bouvy, B. and Poncet, C. (2015).
Long-term prospective evaluation of intestinal anastomosis using stainless steel staples in 14 dogs
The Canadian Veterinary Journal; 56 (7) 715–722
Full text available
64. Schwartz, Z. and Coolman, B.R. (2018)
Closure of gastrointestinal incisions using skin staples alone and in combination with suture in 29 cats
Journal of Small Animal Practice; 59 (5) 281‐285
Abstract
65. Maxwell, E.A., Dugat, D.R., Waltenburg, M., et al. (2021)
Outcomes of dogs undergoing immediate or delayed surgical treatment for gastrointestinal foreign body obstruction: A retrospective study by the Society of Veterinary Soft Tissue Surgery
Veterinary Surgery 50(1), 177-185
Abstract
66. Power, A.M., Diamond, D.W., Puetthoff, C. (2021)
Laparotomy-Assisted Transoral Foreign Body Retrieval in Small Animals: 10 Cases (2018-2020)
Topics in Companion Animal Medicine 42, Article 100504
Abstract
67. Lopez, D.J., Holm, S.A., Korten, B., et al (2021)
Comparison of patient outcomes following enterotomy versus intestinal resection and anastomosis for treatment of intestinal foreign bodies in dogs
Journal of the American Veterinary Medical Association 258(12), 1378-1385
Abstract
68. Mullen, K.M., Regier, P.J., Fox-Alvarez, W.A., et al (2021)
Evaluation of intraoperative leak testing of small intestinal anastomoses performed by hand-sewn and stapled techniques in dogs: 131 cases (2008–2019)
Journal of the American Veterinary Medical Association 258(9), 991-998
Abstract
69. Miles, S., Gaschen, L., Presley, T., et al. (2021)
Influence of repeat abdominal radiographs on the resolution of mechanical obstruction and gastrointestinal foreign material in dogs and cats
Veterinary Radiology and Ultrasound 62(3), 282-288
Abstract
70. Mullen, K.M., Regier, P.J., Waln, M., et al. (2021)
Ex vivo comparison of leak testing of canine jejunal enterotomies: Saline infusion versus air insufflation
Veterinary Surgery 50(6), 1257-1266
Abstract
71. Culbertson, TF, Smeak, DD, Pogue, JM, Vitt, MA, Downey, AC. (2021)
Intraoperative surgeon probe inspection compared to leak testing for detecting gaps in canine jejunal continuous anastomoses: A cadaveric study
Veterinary Surgery 50(7), 1472- 1482
Abstract
72. Friday, S., Murphy, C., Lopez, D. et al (2021)
Gorilla Glue Ingestion in Dogs: 22 Cases (2005–2019)
Journal of the American Animal Hospital Association 57(3), 121-127
Abstract
73. Culbertson, T.F., Smeak, D.D., Rao, S. (2021)
Volume of saline (0.9% NaCl) solution required to reach maximum peristaltic pressure in cadaveric intact jejunal specimens from dogs of various sizes
American Journal of Veterinary Research 82(12), 988-995
Abstract
74. Hoffman, C.L., Mastrocco, A., Drobatz,K.J. et al. (2022)
Retrospective evaluation of gastrointestinal foreign bodies and presurgical predictors for enterectomy versus enterotomy in dogs (2013-2016): 82 cases
Journal of Veterinary Emergency and Critical Care 32(1), 98-105
Abstract
75. Quitzan, J.G., Singh, A., Beaufrère, H., et al. (2022)
Influence of staple line number and configuration on the leakage of small intestinal functional end-to-end stapled anastomosis: An ex vivo study.
Veterinary Surgery 51(5), 781-787
Abstract
76. Duffy, D.J., Chang, Y.J., & Moore, G.E. (2022)
Influence of crotch suture augmentation on leakage pressure and leakage location during functional end-to-end stapled anastomoses in dogs.
Veterinary surgery 51(4), 697-705
Full text available
77. Demars, C., Boland, L., Minier, K. (2023)
Surgical removal of intestinal foreign bodies using a laparotomy-assisted endoscopic approach in dogs and cats and comparison with enterotomy.
Journal of Small Animal Practice 64(1), 43-50
Abstract
78. Sahagian, M.J., Mastrocco, A., Weltman, J.G., et al. (2023)
Retrospective analysis of the use of canine-specific albumin in 125 critically ill dogs.
Journal of Veterinary Emergency and Critical Care 33(2), 192-200
Abstract
79. Silvestre Sombrio, M., Mai, W., Buch, D., et al. (2023)
Accuracy and reliability of tele-ultrasonography in detecting gastrointestinal obstruction in dogs and cats.
Journal of Small Anim Practice 64(6), 367-374
Abstract
80. Mullen, K.M., Regier, P.J., Fox-Alvarez, W.A., et al. (2023)
A quantitative evaluation of the effect of foreign body obstruction and enterectomy technique on canine small intestinal microvascular health.
Veterinary Surgery 52(4), 554-563
Abstract
81. Gollnick, H.R., Schmiedt, C.W., Wallace, M.L., et al. (2023)
Retrospective evaluation of surgical treatment of linear and discrete gastrointestinal foreign bodies in cats: 2009-2021.
Journal of Feline Medicine and Surgery 25(6), 1-5
Full text available
82. Corrick, J. (2023)
Is radiography or ultrasonography superior at detecting intestinal obstructions in dogs with acute abdominal signs?
Veterinary Evidence 8(2). https://doi.org/10.18849/ve.v8i2.483
Full text available
83. Crinò, C., Humm, K. and Cortellini, S. (2023)
Conservative management of metallic sharp-pointed straight gastric and intestinal foreign bodies in dogs and cats: 17 cases (2003-2021)
Journal of Small Animal Practice 64, 522–526
Full text available
84. Puzio CE, Rudloff E, Pigott AM.
Delay of definitive care in cats and dogs with gastrointestinal foreign body obstruction following antiemetic administration: 537 cases (2012-2020)
Journal of Veterinary Emergency and Critical Care 33(4):442-446
Abstract
85. Terradas Crespo, E., Martin, L.G., Davidow, E.B. (2023)
Retrospective evaluation of indications, transfusion protocols, and acute transfusion reactions associated with the administration of lyophilized canine albumin: 53 cases (2009-2020).
Journal of Veterinary Emergency and Critical Care 33(5), 567-576
Full text available
86. Shanaman, M.M., Schwarz, T., Gal, A., et al. (2013)
Comparison between survey radiography, B-mode ultrasonography, contrast-enhanced ultrasonography and contrast-enhanced multi-detector computed tomography findings in dogs with acute abdominal signs.
Veterinary Radiology and Ultrasound 54(6), 591-604
Abstract
87. Lozano, B.A., Yankin, I., Perry, S., et al. (2023)
Acid-base and electrolyte evaluation in dogs with upper GI obstruction: 115 dogs (2015-2021).
Journal of Small Animal Practice 64(11), 696-703
Abstract
88. Henke, C.S., Beal, M.W., Walton, R.A.L., et al. (2023)
Retrospective evaluation of the clinical course and outcome of zinc toxicosis due to metallic foreign bodies in dogs (2005-2021): 55 cases.
Journal of Veterinary Emergency and Critical Care 33(6), 676-684
Full text available
89. Craft, E.M., Powell, L.L. (2012)
The use of canine-specific albumin in dogs with septic peritonitis.
Journal of Veterinary Emergency and Critical Care 22(6), 631-9
Abstract
90. Enders, B., Musulin, S., Holowaychuk, M., et al. (2018)
Repeated infusion of lyophilized canine albumin safely and effectively increases serum albumin and colloid osmotic pressure in healthy dogs.
Journal of Veterinary Emergency and Critical Care 28(S1), S5
Abstract
91. Mathews, K.A., Barry, M. (2005)
The use of 25% human serum albumin: outcome and efficacy in raising serum albumin and systemic blood pressure in critically ill dogs and cats.
Journal of Veterinary Emergency and Critical Care 15, 110- 118
Abstract
92. Lyophilized canine albumin, 5gr, datasheet.
Animal Blood Resources International, Stockbridge, MI
Full text available
93. Peterson, K.L., Hardy, B.T., Hall, K. (2013)
Assessment of shock index in healthy dogs and dogs in hemorrhagic shock.
Journal of Veterinary Emergency and Critical Care 23(5), 545-550
Abstract
94. Yuen, F., Dennison, S. (2024)
Radiographic identification of challenging gastrointestinal tract foreign bodies: a descriptive study of how appearance varies in air versus water to aid interpretation.
American Journal of Veterinary Research 85(7)
Full text available
95. Costello, S., McRae, B., Olive, M., et al. (2024)
Stapled enterectomy reduces surgical time when compared with sutured enterectomy: a retrospective review of 54 cats.
Journal of Feline Medicine and Surgery 26(9), 1098612X241264723
Full text available
96. Carrillo, A.J., McCord, M.A., Dickerson, V.M. (2024)
Clinical features and outcomes of dogs with attempted medical management for discrete gastrointestinal foreign material: 68 cases (2018-2023).
Journal of the American Veterinary Medical Association 262(9), 1251-1258
Full text available
97. Miller, A.K., Regier, P.J., Ham, K.M., et al. (2024)
Linear and discrete foreign body small intestinal obstruction outcomes, complication risk factors, and single incision red rubber catheter technique success in cats.
Veterinary Surgery 53(7), 1256-1265
Abstract
98. Cola, V., Ferrari, C., Del Magno, S. (2024)
Laparotomy-assisted endoscopic removal of gastrointestinal foreign bodies: Evaluation of this technique and postoperative recovery in dogs and cats.
Veterinary Surgery 53(7), 1266-1276
Full text available
99. Genoni, S., Cinti, F., Pilot, M., et al. (2024)
Stapled functional end-to-end intestinal anastomosis with endovascular gastrointestinal anastomosis staplers in cats and small dogs.
Journal of Small Animal Practice 65(11), 799-806
Abstract
100. Rafael, P., Soulé, C., Sériot, P., et al. (2024)
Evaluation of early and systematic ultrasound examination to determine postoperative dehiscence after small intestinal surgery (114 cases in dogs and cats).
Journal of the American Veterinary Medical Association 263(1), 1–10
Full text available
101. Carcéles, A.F., Degani, M., Soler, C., et al. (2025).
Veterinary Enhanced Recovery After Surgery (Vet-ERAS) Program in Dogs Undergoing Emergency Laparotomy.
Veterinary Sciences 12, 377.
Full text available
102. Prettegiani, B. & Maritato, K. (2025).
Comparison of removal of intestinal foreign bodies using orogastric retrieval techniques versus gastrotomies in dogs and cats.
Journal of Small Animal Practice 66(4), 243–247.
Abstract
103. Anatolitou, A., Markou, M. (2025).
Comparative efficacy of gambee and single interrupted suture patterns in reducing complications after canine enterotomy.
Veterinary Evidence 10(3)
Full text available
How this topic was developed
Primary search terms
- title:((“foreign body”)) AND title:(((dog or dogs or canine or canines) OR (cat or cats or feline or feline) OR (“small animals”))) AND yr:[1989 TO 2020]
- title:(intestinal AND (“foreign body”) OR enterotomy) AND title:(((dog or dogs or canine or canines) OR (cat or cats or feline or feline) OR (“small animals”))) AND yr:[1989 TO 2020]
- title:(intestinal AND (“foreign body”) OR enterotomy) AND title:(((dog or dogs or canine or canines) OR (cat or cats or feline or feline) OR (“small animals”))) AND yr:[1950 TO 2020]
- title:(enterotomy) AND title:(((dog or dogs or canine or canines) OR (cat or cats or feline or feline) OR (“small animals”))) AND yr:[1989 TO 2020]
- title:((intestinal OR intestine) AND surgery) AND title:(((dog or dogs or canine or canines) OR (cat or cats or feline or feline) OR (“small animals”))) AND yr:[1989 TO 2020]
Contributors
Writers
- Zoë Coker BSc (Hons) CertGP (EM&S) BVM&S MRCVS
Specialist reviewers
- Sophie Adamantos BVSc CertVA DACVECC DipECVECC MRCVS FHEA
- Nicola Kulendra BVetMed CertVDI DipECVS MRCVS
Disclaimer
See our disclaimer page here for more information.